lpetrich
Contributor
Mathematicians Have Proposed a New Structure to The Periodic Table noting Formal structure of periodic system of elements | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences
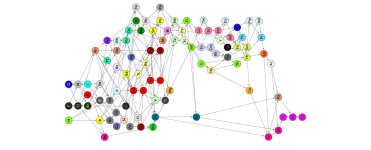
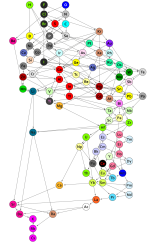
 Alternative periodic tables shows other ones, but none of them are the sort of mess that this new one is.
Alternative periodic tables shows other ones, but none of them are the sort of mess that this new one is.
But I cannot make any sense out of the arrangement. It looks like a big fat mess without rhyme or reason. So I'm sticking with the arrangement that is now traditional."We have investigated almost 5,000 substances consisting of two elements in different proportions. We then looked for similarities within this data. For example, sodium and lithium are similar because they combine with the same elements in the same proportions (e.g. with oxygen or chlorine, bromine, and iodine). We thus found patterns we can use to classify the elements."
This system, based on chemical bonds, rearranges the elements in a new way. Some elements remain grouped together, such as halogens, because they bond the same way; but others are separated, like silicon and carbon, which, when bonded, form very different compounds.