-
Features
-
Friends of IIDBFriends Recovering from Religion United Coalition of Reason Infidel Guy
Forums Council of Ex-Muslims Rational Skepticism
Social Networks Internet Infidels Facebook Page IIDB Facebook Group
The Archives IIDB Archive Secular Café Archive
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Why are depictions of electrons round?
- Thread starter none
- Start date
beero1000
Veteran Member
why are depictions of electrons, protons, and neutrons showing them as round?
Because that's an easy way to visualize particles. I believe that scattering experiments have shown that they usually act like they are round, but may fluctuate to non-spherical shapes.
Proton shapes:
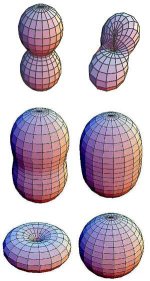
ryan
Veteran Member
- Joined
- Jun 26, 2010
- Messages
- 4,668
- Location
- In a McDonalds in the q space
- Basic Beliefs
- a little of everything
Those would be electron orbitals. The second row looks like the s1 in phase molecular bond orbitals of 2 electrons from 2 covalently bonded hydrogen atoms.
The electron, if actually just a point particle, would be round in the sense that all points on the point are equal distances from its center (zero distance).
The electron, if actually just a point particle, would be round in the sense that all points on the point are equal distances from its center (zero distance).
fast
Contributor
why are depictions of electrons, protons, and neutrons showing them as round?
This is just a shot in the dark here, but for some reason or another, I would have figured that an attempt to accurately depict the shape of two of the three would be reasonably realistic. It would be (I would have guessed) more likely to approximate the shape of protons and neutrons while substantially more difficult to guess the shape of an electron.
That is because the sheer size of the electron puts it on the other side of that scientific bridge cutting off the directly observed from the indirectly observed. In other words, protons and neutrons are so much comparitavely larger that we have had the opportunity to scientifically observe them in fact while the elusively spotable electron is so tiny that we are left to infer their existence by the effect on things we actually can directly observe.
braces_for_impact
Veteran Member
We're trying to make sense of something that lies outside all of our practical experience. I've met may people that think the Bohr model is actually what atoms look like, basically a little solar system of sorts. I don't think when they're teaching this they emphasise that enough. So, the best we can do when describing an atom is to compare it to something we DO understand better.
bilby
Fair dinkum thinkum
- Joined
- Mar 6, 2007
- Messages
- 35,754
- Gender
- He/Him
- Basic Beliefs
- Strong Atheist
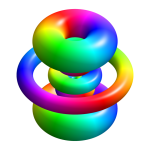
Generally no attempt is made to depict the 'actual' shape, and a sphere is just arbitrarily chosen. In some situations, there is an attempt to depict the shape of the probability distribution of a particle's position; this can take a number of shapes, depending on the environment, as beero1000's nice pictures of protons above illustrates. As there is a non-zero amplitude for a particle to be found almost anywhere in the universe, what is shown is usually the boundary of the space in which the probability density (the modulus squared of the amplitude) is above a chosen threshold; This is a picture of the probability distribution of an electron in a Hydrogen atom (source):

My chemistry professor said, "Electrons are little mushy balls."
I prefer to think of electrons as a woman you met on the internet, but will never meet in real life. You can imagine the space she occupies, and she can send you a picture, but you'll never be really certain.
I prefer to think of electrons as a woman you met on the internet, but will never meet in real life. You can imagine the space she occupies, and she can send you a picture, but you'll never be really certain.
Malintent
Veteran Member
Those would be electron orbitals. The second row looks like the s1 in phase molecular bond orbitals of 2 electrons from 2 covalently bonded hydrogen atoms.
The electron, if actually just a point particle, would be round in the sense that all points on the point are equal distances from its center (zero distance).
a point cannot be round any more than a circle can have volume. However, an electron is not a point. It isn't even a material 'thing'... it is a component of a wave function. electrons exist the same way the vertex of a curve exists.... conceptually.
fast
Contributor
Awe man, I'm never gonna get this stuff straight.Those would be electron orbitals. The second row looks like the s1 in phase molecular bond orbitals of 2 electrons from 2 covalently bonded hydrogen atoms.
The electron, if actually just a point particle, would be round in the sense that all points on the point are equal distances from its center (zero distance).
a point cannot be round any more than a circle can have volume. However, an electron is not a point. It isn't even a material 'thing'... it is a component of a wave function. electrons exist the same way the vertex of a curve exists.... conceptually.
Kharakov
Quantum Hot Dog
Awe man, I'm never gonna get this stuff straight.a point cannot be round any more than a circle can have volume. However, an electron is not a point. It isn't even a material 'thing'... it is a component of a wave function. electrons exist the same way the vertex of a curve exists.... conceptually.
That isn't even possible, with all the curves in the underlying structure of the universe. One thing about science, it looks at the flow of information in the universe as if the information flow (energy flow) was an independent thing, for convenience. The idea of an electron as an individual thing (which they can appear to be!) is like one of your thoughts being an individual thing- the thought of 1+1=2 is an individual thing, and at the same time exists as part of a whole consciousness.
Likewise, the electron exists as a part (icle), and as part of the whole (wave
 ).
).fromderinside
Mazzie Daius
- Joined
- Oct 6, 2008
- Messages
- 15,945
- Basic Beliefs
- optimist
Space, point, frequency, anything else?
Generally no attempt is made to depict the 'actual' shape, and a sphere is just arbitrarily chosen. In some situations, there is an attempt to depict the shape of the probability distribution of a particle's position; this can take a number of shapes, depending on the environment, as beero1000's nice pictures of protons above illustrates. As there is a non-zero amplitude for a particle to be found almost anywhere in the universe, what is shown is usually the boundary of the space in which the probability density (the modulus squared of the amplitude) is above a chosen threshold; This is a picture of the probability distribution of an electron in a Hydrogen atom (source):
View attachment 11521
And the pretty colors show which color quarks are part of the electron at each point

(Lick it and you'll get the flavors too. Although I don't much care for strange....)
J842P
Veteran Member
Those would be electron orbitals. The second row looks like the s1 in phase molecular bond orbitals of 2 electrons from 2 covalently bonded hydrogen atoms.
The electron, if actually just a point particle, would be round in the sense that all points on the point are equal distances from its center (zero distance).
Those are *not* electron orbitals. Read the linked paper.
ryan
Veteran Member
- Joined
- Jun 26, 2010
- Messages
- 4,668
- Location
- In a McDonalds in the q space
- Basic Beliefs
- a little of everything
Those would be electron orbitals. The second row looks like the s1 in phase molecular bond orbitals of 2 electrons from 2 covalently bonded hydrogen atoms.
The electron, if actually just a point particle, would be round in the sense that all points on the point are equal distances from its center (zero distance).
a point cannot be round any more than a circle can have volume. However, an electron is not a point. It isn't even a material 'thing'... it is a component of a wave function. electrons exist the same way the vertex of a curve exists.... conceptually.
An electron is material.

