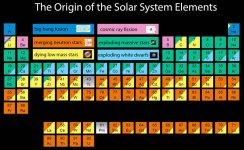
Interesting. All those elements are found naturally on our earth (right?!), which implies that we have had all those events happen in the vicinity of our solar system at some point in the past. That is, a neutron star collision, a massive star exploding, a white dwarf exploding and a dying low mass star. Given our solar system is only around 5 billion years old, and the universe is around 14 billion, that's a lot of stellar activity that happened here in 9 billion years. Is that typical or would that be considered unusual? If its pretty rare, then I would think life in the universe (as we know it anyway) would be rare as well, as a huge chunk of the elements, (even in trace amounts), in the table are critical to our being here. Or am I off base on something?
Well, most of the Earth is made up of just a few elements. oxygen, silicon, aluminium, iron, calcium, magnesium, potassium and sodium between them make up about 97% of the earth by mass, with all the rest in that 3%. Of course, 3% of an entire planet is still quite a lot of stuff on human scales, but on solar system scales, the Earth itself is a tiny bit of slag left over from the sun's formation. Up to about 5 significant digits, there isn't anything in the solar system except the sun, and there's nothing in the sun except Hydrogen and a bit of Helium. Jupiter is the only significant extra-solar impurity; the rocky stuff - mostly oxygen, silicon, aluminium and iron - that makes up the four inner planets, the asteroid belt, and a scattering of other minor planets and debris adds up to bugger all as a fraction of the Solar System. Of course we tend to think of the slag of 'metals' (elements other than H and He) as important, but only because they include us and our home planet.
As to being critical to our being here, all we really need in significant quantities are hydrogen, carbon, oxygen, nitrogen and a trace of phosphorous and sulphur. The other elements are not needed in more than tiny quantities when making plants or animals.